
A Basic Guide To The Electronic Common Technical Document

Drug Master Files Dmfs Electronic Submission Deadline Extended

Fda And Electronic Common Technical Document By Compliance Global

Electronic Common Technical Document Ectd Update Jon Clark Ich

Figure 2 From The Electronic Common Technical Document Ectd

Guidance Document Preparation Of Drug Regulatory Activities In

Submissions Management Software Publish Ectd Submissions

Globalsubmit Review For Regulatory Review Submissions Management

Acorn Regulatory 60 Seconds About Ectd Electronic Common
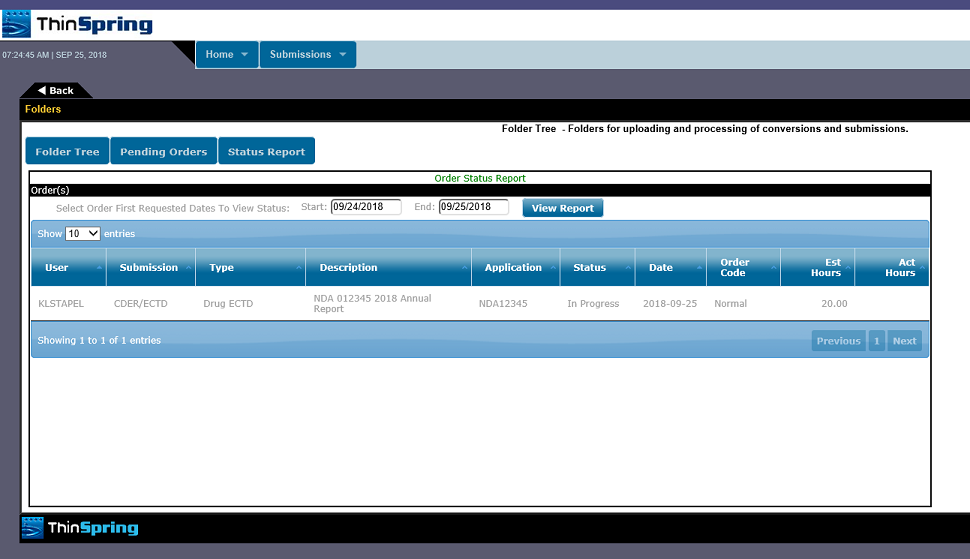
Fda And Hc Submissions Ectd Nda Anda Cbe Electronic Common

Pdf The History Of Electronic Regulatory Submissions

Electronic Common Technical Document Wikipedia

Uae Ministry Of Health Prevention Implements Electronic Common

Health Canada Implements Special Requirements For Electronic

Guidance Document Preparation Of Drug Regulatory Activities In

Turaskills Shares Tip For Writing Ctd Ectd Comparison Of Paper

Turaskills Shares Tip For Writing Sofware Of Ectd Ectd
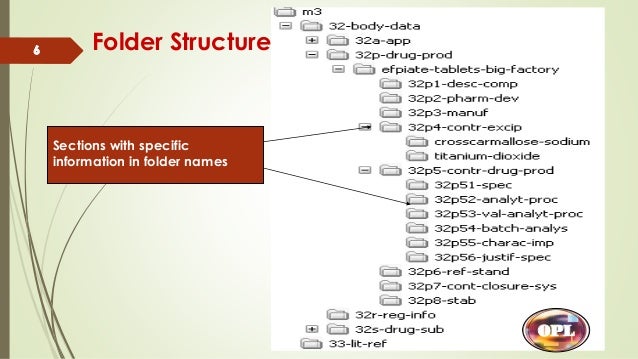
Electronic Common Technical Document Ectd

Electronic Common Technical Document Ectd Update Jon Clark Ich

Required Ectd Submissions As Of May 5 2017 Are You Ready
Post a Comment for "Electronic Common Technical Document Ectd"